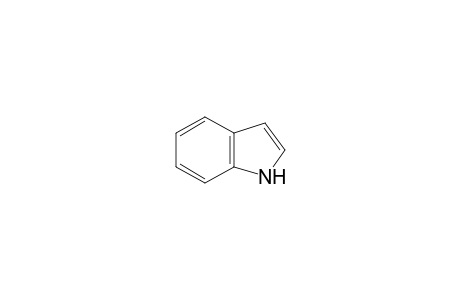
Indole
Compound with spectra: 165 NMR, 16 FTIR, 2 Raman, 42 MS (GC), and 2 Near IR
| SpectraBase Compound ID | BGv5NPMT2gH |
|---|---|
| InChI | InChI=1S/C8H7N/c1-2-4-8-7(3-1)5-6-9-8/h1-6,9H |
| InChIKey | SIKJAQJRHWYJAI-UHFFFAOYSA-N |
| Mol Weight | 117.15 g/mol |
| Molecular Formula | C8H7N |
| Exact Mass | 117.057849 g/mol |
| Copyright | Database Compilation Copyright © 2023-2024 John Wiley and Sons, Inc. Copyright © 2023-2024 John Wiley and Sons, Inc., Portions Copyright Wiley-VCH GmbH, Adams Library under license from Diablo Analytical. All Rights Reserved. |
|---|---|
| Source of Spectrum | Adams' Essential Oil Components (GC-MS), Version 4 |
| Copyright | Database Compilation Copyright © 2023-2024 John Wiley and Sons, Inc. Copyright © 2023-2024 John Wiley and Sons, Inc., Portions Copyright Wiley-VCH GmbH, Adams Library under license from Diablo Analytical. All Rights Reserved. |
|---|---|
| Source of Sample | SAFC Cat.no. W259306 |
| Source of Spectrum | Prof. L. Mondello (Chromaleont s.r.l./Univ. Messina, Italy) |
| Copyright | Database Compilation Copyright © 2023-2024 John Wiley and Sons, Inc. Copyright © 2023-2024 John Wiley and Sons, Inc., Portions Copyright Wiley-VCH GmbH, Adams Library under license from Diablo Analytical. All Rights Reserved. |
|---|---|
| Source of Sample | SAFC Cat. #W259306 |
| Source of Spectrum | Prof. L. Mondello (Chromaleont s.r.l./Univ. Messina, Italy) |
| Copyright | Database Compilation Copyright © 2023-2024 John Wiley and Sons, Inc. Copyright © 2023-2024 John Wiley and Sons, Inc., Portions Copyright Wiley-VCH GmbH, Adams Library under license from Diablo Analytical. All Rights Reserved. |
|---|---|
| Source of Spectrum | Chemical Concepts, A Wiley Division, Weinheim, Germany |
- 1H-indole
- 1-Benzazole
- Indol
- 2,3-Benzopyrrole
- 1-Azaindene
- Ketole
- Benzopyrrole
- 1-BENZO/B/PYRROLE
- BENZO/B/PYRROLE
- 1-INDOLE
- SIKJAQJRHWYJAI-UHFFFAOYSA-N
- REFERENCE
- Benzo-2,3-pyrrole
- 1H-Benzo[b]pyrrole
This compound is available in the following databases:
Mass Spectra of Designer Drugs 2024
Author: Peter Rösner
This library enables fast and reliable identification of the latest illegal designer drugs. It is updated annually. Learn more.
KnowItAll NMR Spectral Library
Author: Wiley
The KnowItAll NMR Spectral Library offers a comprehensive collection of NMR reference spectra, including NMR, CNMR, and XNMR, and covers a wide range of compounds including organics, polymers, monomers, metabolites, and more. Learn more.
KnowItAll IR, Raman, and UV-Vis Spectral Libraries
Author: Wiley
The KnowItAll IR, Raman, and UV-Vis spectral libraries offer access to the world's largest collection of IR, Raman, and UV-Vis spectra, including the renowned Sadtler spectra. The libraries include a wide range of compounds from pure organics to industrial compounds. Learn more.
KnowItAll Mass Spectral Library
Author: Wiley
The KnowItAll Mass Spectral Library offers a comprehensive collection of mass spectra, including the renowned Wiley Registry, and access to a wide range of compounds including pure organics, drugs, steroids, additives, geochemicals, petrochemicals, biomarkers, and more. Learn more.
Maurer, Meyer, Pfleger, Weber: GC-MS Library of Drugs, Poisons, and Their Metabolites 6th Edition
Author: Hans H. Maurer, Markus Meyer, Karl Pfleger, Armin A. Weber
Identification of trace drugs, poisons, and pollutants requires broad coverage of specific compound classes: metabolites, acetylated, methylated, trimethylsilylated, trifluoroacetylated, pentafluoropropionylated, and heptafluorobutyrylated compounds. Learn more.
Food, Flavors, Fragrances, and Related Compounds: GC-MS Library
Author: Wiley
With Wiley's Food, Flavors, Fragrances, and Related Compounds GC-MS Library, get access to GC-MS spectra with focused compound coverage including essential oils, lipids, volatile organic compounds, terpenes, herbicides, insecticides, and other compounds of importance for food, flavor, fragrance, and cannabis applications. Learn more.
Wiley Registry of Mass Spectral Data 2023
Author: Wiley
The foundation library for any analytical laboratory running mass spectrometry, the Wiley Registry provides the broadest coverage available in any mass spectral library Learn more.