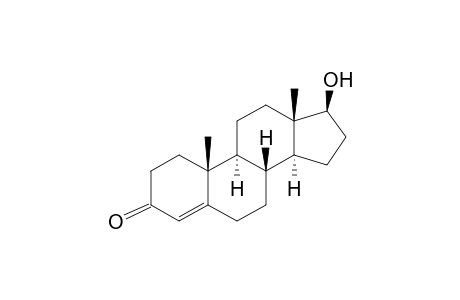
Testosterone
Compound with spectra: 10 NMR, 4 FTIR, 1 Raman, and 8 MS (GC)
| SpectraBase Compound ID | 94P1FLQ5KFm |
|---|---|
| InChI | InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 |
| InChIKey | MUMGGOZAMZWBJJ-DYKIIFRCSA-N |
| Mol Weight | 288.43 g/mol |
| Molecular Formula | C19H28O2 |
| Exact Mass | 288.20893 g/mol |
| Enantiomer InChIKey | MUMGGOZAMZWBJJ-MGYGNFHQSA-N |
| Copyright | Copyright © 2020-2024 John Wiley & Sons, Inc. All Rights Reserved. |
|---|---|
| Source of Spectrum | CAY-2023-426-0 |
| Catalog Number | ISO60154 |
- 4-Androsten-17beta-ol-3-one
- 17-BETA-HYDROXY-ANDROST-4-EN-3-ONE,(TESTOSTERON)
- TESTOSTERONE;17-BETA-HYDROXY-ANDROST-4-EN-3-ONE
- ANDROST-4-EN-3-ONE, 17B-HYDROXY-,
- MERTESTATE
- ANDROLIN
- TESLEN
- 17BETA-HYDROXYANDROST-4-EN-3-ONE
- ANDROST-4-EN-3-ONE, 17-HYDROXY-, (17beta)-
- 17-Hydroxyandrost-4-en-3-one
- 17.BETA.-HYDROXY-ANDROST-4-ENE-3-ONE
- ANDROST-4-ENE-17.BETA.-OL-3-ONE
- Androderm
- Testoderm
- (8R,9S,10R,13S,14S,17S)-17-Hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-\rdodecahydrocyclopenta[a]phenanthren-3-one
- (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one
- (1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0(2,7).0(11,15)]heptadec-6-en-5-one
- TESTOVIRON
2,3,4-13C3-Testosterone
Testosterone
17-Hydroxyandrost-4-en-3-one
[16,16,17-Trideuterio]-17.alpha.-hydroxyandrost-4-en-3-one
Epitestosterone
(8R,9S,10R,13S,14S,17S)-16,16,17-trideuterio-10,13-dimethyl-17-oxidanyl-1,2,6,7,8,9,11,12,14,15-decahydrocyclopenta[a]phenanthren-3-one
17-Hydroxyandrost-4-en-3-one
Testosterone
(17-alpha)-17-Hydroxyandrost-4-en-3-one
3-METHYLENE-ANDROST-4-EN-17-BETA-OL
21-Hydroxypregn-4-en-3-one
Androst-4-en-3-one, 17-methyl-
17-METHYLANDROST-4-EN-3-ONE
17-ALPHA-CHLOROANDROST-4-EN-3-ONE
Pregn-4-en-3-one, 20-methyl-
21-Hydroxypregn-4-en-3-one, carbonate (2:1)
17.alpha.,19-dihydroxyandrost-4-en-3-one
17-BETA-HYDROXY-2-OXA-ANDROST-4-EN-3-ONE
13-(1',3'-Dioxolan-2'-yl)-3-oxo-16,17-seco-17-nor-androst-5-ene-16-nitrile
| Title | Journal or Book | Year |
|---|---|---|
| An unusual ring—A opening and other reactions in steroid transformation by the thermophilic fungus Myceliophthora thermophila | The Journal of Steroid Biochemistry and Molecular Biology | 2009 |
| Predominant allylic hydroxylation at carbons 6 and 7 of 4 and 5-ene functionalized steroids by the thermophilic fungus Rhizomucor tauricus IMI23312 | The Journal of Steroid Biochemistry and Molecular Biology | 2008 |
| Metabolism of anabolic steroids in humans: Synthesis of 6β-hydroxy metabolites of 4-chloro-1,2-dehydro-17α-methyltestosterone, fluoxymesterone, and metandienone | Steroids | 1995 |
| Microbial transformation of steroids: Contribution to 14α-hydroxylations | Steroids | 1995 |
| 13C n.m.r. spectra of steroids —a survey and commentary | Organic Magnetic Resonance | 1977 |
This compound is available in the following databases:
Mass Spectra of Physiologically Active Substances - including drugs, steroid hormones,and endocrine disruptors 2011
Author: Parr, Maria Kristina; Opfermann, Georg; Schänzer, Wilhelm; Makin, Hugh L. J.
Fast and reliable identification of trace steroids requires an up-to-date library covering most known and newly emerging steroids. It is updated annually. Learn more.
Mass Spectra of Designer Drugs 2024
Author: Peter Rösner
This library enables fast and reliable identification of the latest illegal designer drugs. It is updated annually. Learn more.
KnowItAll NMR Spectral Library
Author: Wiley
The KnowItAll NMR Spectral Library offers a comprehensive collection of NMR reference spectra, including NMR, CNMR, and XNMR, and covers a wide range of compounds including organics, polymers, monomers, metabolites, and more. Learn more.
KnowItAll IR, Raman, and UV-Vis Spectral Libraries
Author: Wiley
The KnowItAll IR, Raman, and UV-Vis spectral libraries offer access to the world's largest collection of IR, Raman, and UV-Vis spectra, including the renowned Sadtler spectra. The libraries include a wide range of compounds from pure organics to industrial compounds. Learn more.
KnowItAll Mass Spectral Library
Author: Wiley
The KnowItAll Mass Spectral Library offers a comprehensive collection of mass spectra, including the renowned Wiley Registry, and access to a wide range of compounds including pure organics, drugs, steroids, additives, geochemicals, petrochemicals, biomarkers, and more. Learn more.
Maurer, Meyer, Pfleger, Weber: GC-MS Library of Drugs, Poisons, and Their Metabolites 6th Edition
Author: Hans H. Maurer, Markus Meyer, Karl Pfleger, Armin A. Weber
Identification of trace drugs, poisons, and pollutants requires broad coverage of specific compound classes: metabolites, acetylated, methylated, trimethylsilylated, trifluoroacetylated, pentafluoropropionylated, and heptafluorobutyrylated compounds. Learn more.
Wiley Registry of Mass Spectral Data 2023
Author: Wiley
The foundation library for any analytical laboratory running mass spectrometry, the Wiley Registry provides the broadest coverage available in any mass spectral library Learn more.
Maurer, Meyer, Helfer, Weber: LC-HR-MS & MS Library of Drugs, Poisons, and Their Metabolites
Author: Hans H. Maurer, Markus Meyer, Andreas G. Helfer, Armin A. Weber
Developed by toxicologist Hans H. Maurer and his team, the Maurer/Meyer/Helfer/Weber LC-HR-MS/MS Library of Drugs, Poisons, and Their Metabolites consists of spectra for parent drugs or poisons, and their metabolites or artifacts, in various compound classification groups. This metabolite-based library helps minimize the risk of false negative LC-MS results. Learn more.