| SpectraBase Spectrum ID |
LcxEGKjzhJf |
| Name |
FIBF |
| Source of Sample |
Cayman Chemical Company |
| Catalog Number |
Free base of 19313 |
| Lot Number |
Free base of 0481306-14 |
| Accessory |
DurasamplIR II |
| Acronyms |
4-FIBF; p-FIBF; para-FIBF; 4-Fluoro iBF; p-Fluoro iBF; para-Fluoro iBF |
| Copyright |
Copyright © 2019-2025 John Wiley & Sons, Inc. All Rights Reserved. |
| DEA Schedule |
I |
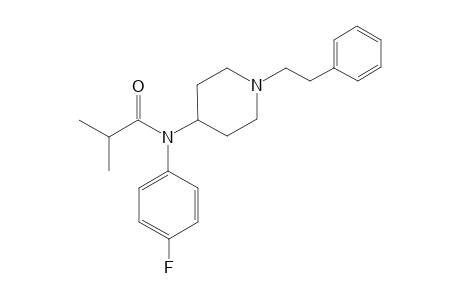
| Formula |
C23H29FN2O |
| IUPAC Name |
N-(4-Fluorophenyl)-2-methyl-N-[1-(2-phenylethyl)-4-piperidinyl]propanamide |
| InChI |
InChI=1S/C23H29FN2O/c1-18(2)23(27)26(21-10-8-20(24)9-11-21)22-13-16-25(17-14-22)15-12-19-6-4-3-5-7-19/h3-11,18,22H,12-17H2,1-2H3 |
| InChIKey |
OZDOSQNUJIXEOR-UHFFFAOYSA-N |
| Instrument Name |
Bio-Rad FTS |
| SMILES |
C(=O)(N(C1CCN(CC1)CCc1ccccc1)c1ccc(cc1)F)C(C)C |
| Sample Preparation Procedure |
The HCl salt was dissolved in 0.5 mL water in a test tube, and 1 drop of 30% NaOH solution was added, causing the clear solution to turn cloudy white. 1 mL dichloromethane was added to the test tube, which was shaken thoroughly using a vortex mixer. The test tube was spun in a centrifuge to separate the organic and aqueous layers. The bottom organic layer was retrieved with a pipette and placed in another clean test tube. Most of the solvent was evaporated from the tube using a flow of nitrogen gas, and the remaining solution was used to create a film on the diamond window for ATR-IR. |
| Solvent |
Dichloromethane |
| Source of Spectrum |
Forensic Spectral Research |
| Synonyms |
4-Fluoroisobutyryl fentanyl; p-Fluoroisobutyryl fentanyl; para-Fluoroisobutyryl fentanyl |
| Technique |
ATR-Film |