| SpectraBase Spectrum ID |
LP8hGPvJCT6 |
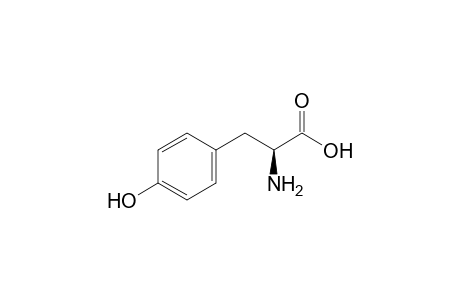
| Name |
L-Tyrosine |
| Acquisition Mode |
SIMULTANEOUS |
| CAS Registry Number |
140-43-2
1991-85-1
46209-14-7
55520-40-6
60-18-4 |
| ChEBI ID |
17895 |
| Comments |
saturated N/A L-tyrosine - vendor: Sigma t3754; Solvent: D2O; Temperature=298 K, pH=10.5; NMR Reference: 500 uM DSS; Bruker DMX 400MHz
(Data collected by Madison Metabolomics Consortium) |
| Copyright |
Database Compilation Copyright © 2021-2025 John Wiley & Sons, Inc. All Rights Reserved. |
| Data Source |
Madison Metabolomics Consortium |
| Formula |
C9H11NO3 |
| IUPAC Name |
(2S)-2-amino-3-(4-hydroxyphenyl)propanoic acid |
| InChI |
InChI=1S/C9H11NO3/c10-8(9(12)13)5-6-1-3-7(11)4-2-6/h1-4,8,11H,5,10H2,(H,12,13)/t8-/m0/s1 |
| InChIKey |
OUYCCCASQSFEME-QMMMGPOBSA-N |
| KEGG Compound ID |
C00082 |
| KEGG Pathways |
PATH: map00350 Tyrosine metabolism
PATH: map00400 Phenylalanine, tyrosine and tryptophan biosynthesis
PATH: map00401 Novobiocin biosynthesis
PATH: map00950 Alkaloid biosynthesis I
PATH: map00970 Aminoacyl-tRNA biosynthesis
PATH: map01055 Biosynthesis of vancomycin group antibiotics
PATH: map04916 Melanogenesis |
| PubChem Compound ID |
6057 |
| SMILES |
C1=CC(=CC=C1CC(C(=O)O)N)O |
| Source File Reference |
bmse000051 |