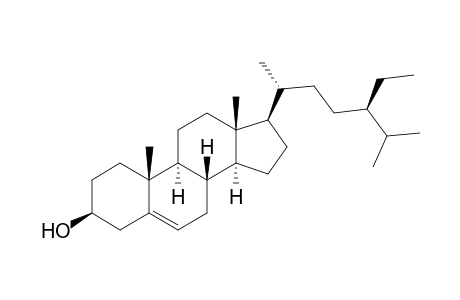
| SpectraBase Compound ID | AtLF3Bk342p |
|---|---|
| InChI | InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 |
| InChIKey | KZJWDPNRJALLNS-VJSFXXLFSA-N |
| Mol Weight | 414.7 g/mol |
| Molecular Formula | C29H50O |
| Exact Mass | 414.386166 g/mol |
13C Nuclear Magnetic Resonance (NMR) Spectrum
View the Full Spectrum for FREE!
The full spectrum can only be viewed using a FREE account.
| SpectraBase Spectrum ID | Kxk1Jqhzjb3 |
|---|---|
| Name | β-sitosterol |
| Acquisition Mode | SIMULTANEOUS |
| CAS Registry Number | 83-46-5 |
| ChEBI ID | 27693 |
| Comments | Saturated beta-sitosterol - Sigma-Aldrich Solvent CDCl37.4, temperature 298 K |
| Copyright | Database Compilation Copyright © 2021-2024 John Wiley & Sons, Inc. All Rights Reserved. |
| Data Source | Madison Metabolomics Consortium |
| Formula | C29 H50 O |
| IUPAC Name | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3S,8S,9S,10R,13R,14S,17R)-17-[(1R,4R)-4-ethyl-1,5-dimethyl-hexyl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3S,8S,9S,10R,13R,14S,17R)-17-[(1R,4R)-4-ethyl-1,5-dimethylhexyl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol; (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methyl-heptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| InChI | InChI=1S/C29H50O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h10,19-21,23-27,30H,7-9,11-18H2,1-6H3/t20-,21-,23+,24+,25-,26+,27+,28+,29-/m1/s1 |
| InChIKey | KZJWDPNRJALLNS-VJSFXXLFSA-N |
| KEGG Compound ID | C01753 |
| KEGG Pathways | PATH: ko00100 Biosynthesis of steroids |
| PubChem Compound ID | 222284 |
| SMILES | CCC(CCC(C)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C)C(C)C; CC[C@H](CC[C@@H](C)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O)C)C)C(C)C |
| Source File Reference | bmse000477 |