| SpectraBase Spectrum ID |
GR2wGgFpVRt |
| Name |
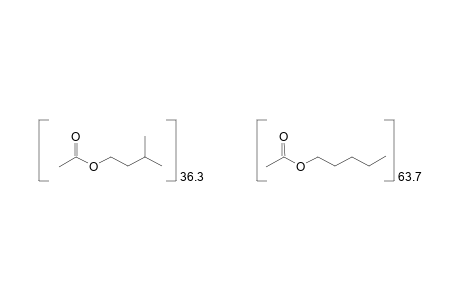
Amyl acetate (mixed isomers: 63.7% amyl, 36.3% isoamyl) |
| Comments |
Computed using HOSE algorithm |
| Copyright |
Copyright © 2024-2025 John Wiley & Sons, Inc. All Rights Reserved. |
| Exact Mass |
13009.937968880 u |
| Formula |
C700H1400O200 |
| InChI |
InChI=1S/99C7H14O2/c36*1-6(2)4-5-9-7(3)8;63*1-3-4-5-6-9-7(2)8/h36*6H,4-5H2,1-3H3;63*3-6H2,1-2H3 |
| InChIKey |
SFOULMWUGTUEKT-UHFFFAOYSA-N |
| Molecular Weight |
13018.700 g/mol |
| Nominal Mass |
13000 u |
| SMILES |
C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)CC.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C.C(CCOC(C)=O)(C)C |