| SpectraBase Spectrum ID |
DxGRCCYxx7M |
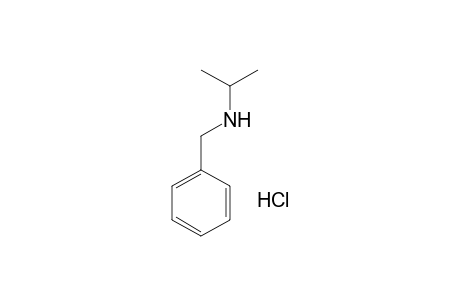
| Name |
N-Isopropylbenzylamine hydrochloride |
| Source of Sample |
Sigma-Aldrich Company LLC |
| Catalog Number |
HCl salt of 136964 |
| Lot Number |
HCl salt of 1378062 |
| Copyright |
Copyright © 2012-2024 John Wiley & Sons, Inc. All Rights Reserved. |
| Formula |
C10H16ClN |
| InChI |
InChI=1S/C10H15N.ClH/c1-9(2)11-8-10-6-4-3-5-7-10;/h3-7,9,11H,8H2,1-2H3;1H |
| InChIKey |
PEIJPMBOGRPKQJ-UHFFFAOYSA-N |
| Instrument Name |
Bio-Rad FTS |
| PubChem Compound ID |
66024 |
| SMILES |
N(Cc1ccccc1)C(C)C.Cl |
| Sample Preparation Procedure |
The free amine was added to a test tube, and a minimum amount of dichloromethane (several drops) was added to completely dissolve the sample. The test tube was then filled halfway with anhydrous diethyl ether or hexane. Hydrochloric acid gas was bubbled through the solution, which led to the precipitation of the HCl salt of amine. The test tube was spun in a centrifuge to separate the HCl salt precipitate from the organic solvent. The organic solvent was removed from the tube, and the precipitate was dried with nitrogen gas. |
| Source of Spectrum |
Forensic Spectral Research |
| Synonyms |
N-Isopropylbenzylamine HCl |
| Technique |
KBr0 |