| SpectraBase Spectrum ID |
3MQSo2PR9yq |
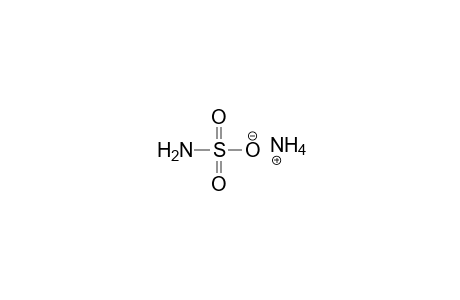
| Name |
AMMONIUM SULFAMATE |
| Source of Sample |
A & S Corporation |
| Chemical Description |
SULFAMIC ACID, MONOAMMONIUM SALT |
| Classification |
MONOMERS - MISCELLANEOUS MONOMERS |
| Compound Type |
Pure |
| Copyright |
Copyright © 1980, 1981-2025 John Wiley & Sons, Inc. All Rights Reserved. |
| InChI |
InChI=1S/H3NO3S.H3N/c1-5(2,3)4;/h(H3,1,2,3,4);1H3 |
| InChIKey |
GEHMBYLTCISYNY-UHFFFAOYSA-N |
| Melting Point |
131C |
| Sample Procedure |
The pyrolysis technique is commonly used in the infrared identification of polymers and results in spectra of both the volatile and non-volatile pyrolyzed fractions the polymer under investigation. It can be used by researchers to identify principal polymer types. The volatile (Vapor Phase) and non-volatile (Condensed Phase) IR spectra resulting from the pyrolysis of this sample are available in SpectraBase as AMMONIUM SULFAMATE - Controlled Pyrolyzate (Vapor Phase) and AMMONIUM SULFAMATE - Controlled Pyrolyzate (Condensed Phase). |
| Technique |
KBr WAFER |