Kaempferol
Compound with spectra: 44 NMR, 3 FTIR, 1 Raman, and 13 MS (GC)
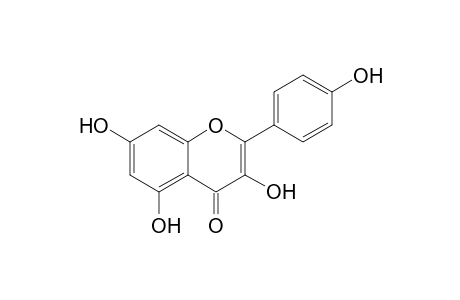
| SpectraBase Compound ID | KgSc5w4uRnA |
|---|---|
| InChI | InChI=1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H |
| InChIKey | IYRMWMYZSQPJKC-UHFFFAOYSA-N |
| Mol Weight | 286.24 g/mol |
| Molecular Formula | C15H10O6 |
| Exact Mass | 286.047738 g/mol |
- 3,5,7,4'-Tetrahydroxy-flavone
- Robigenin
- KAMPFEROL
- KAEMPFEROL;REFERENCE-6
- KAEMPFEROL;3,4',5,7-TETRAHYDROXY-FLAVONE
- 5,7,4'-TRIHYDROXY-FLAVONOL
- 3,4',5,7-TETRAHYDROXYFLAVONE
- KAMPHEROL
- 3,5,7-TRIHYDROXY-2-(4-HYDROXYPHENYL)-4H-1-BENZOPYRAN-4-ONE
- FLAVONE, 3,4*,5,7-TETRAHYDROXY-,
- 2-(4-Hydroxyphenyl)-3,5,7-trihydroxy-4-oxobenzopyran-4-one (kaempferol)
- 4',5,7-Trihydroxyflavonol
- 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-
This compound is available in the following databases:
KnowItAll NMR Spectral Library
Author: Wiley
The KnowItAll NMR Spectral Library offers a comprehensive collection of NMR reference spectra, including NMR, CNMR, and XNMR, and covers a wide range of compounds including organics, polymers, monomers, metabolites, and more. Learn more.
KnowItAll IR, Raman, and UV-Vis Spectral Libraries
Author: Wiley
The KnowItAll IR, Raman, and UV-Vis spectral libraries offer access to the world's largest collection of IR, Raman, and UV-Vis spectra, including the renowned Sadtler spectra. The libraries include a wide range of compounds from pure organics to industrial compounds. Learn more.
KnowItAll Mass Spectral Library
Author: Wiley
The KnowItAll Mass Spectral Library offers a comprehensive collection of mass spectra, including the renowned Wiley Registry, and access to a wide range of compounds including pure organics, drugs, steroids, additives, geochemicals, petrochemicals, biomarkers, and more. Learn more.
Wiley Registry of Mass Spectral Data 2023
Author: Wiley
The foundation library for any analytical laboratory running mass spectrometry, the Wiley Registry provides the broadest coverage available in any mass spectral library Learn more.