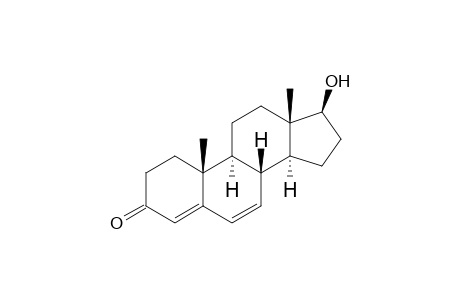
6-Dehydrotestosterone
Compound with spectra: 2 NMR, 2 FTIR, 1 Raman, and 2 MS (GC)
| SpectraBase Compound ID | Ah5W32ScPu2 |
|---|---|
| InChI | InChI=1S/C19H26O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h3-4,11,14-17,21H,5-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 |
| InChIKey | UMDCOKNNLDEKJB-DYKIIFRCSA-N |
| Mol Weight | 286.41 g/mol |
| Molecular Formula | C19H26O2 |
| Exact Mass | 286.19328 g/mol |
| Enantiomer InChIKey | UMDCOKNNLDEKJB-MGYGNFHQSA-N |
- delta 6-Testosterone
- 4 6-Androstadien-17b-ol-3-one
- 17-BETA-HYDROXY-ANDROSTA-4,6-DIEN-3-ONE
- ANDROSTA-4,6-DIEN-17-BETA-OL-3-ONE
- 17.BETA.-HYDROXY-ANDROSTA-4,6-DIENE-3-ONE
- ANDROSTA-4,6-DIENE-17.BETA.-OL-3-ONE
- (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-8,9,10,11,12,13,14,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3(2H)-one
- 17-HYDROXYANDROSTA-4,6-DIEN-3-ONE
4,6-Pregnadien-3-one
(8S,9S,10R,13R,14S,17S)-17-ethyl-10,13-dimethyl-8,9,11,12,14,15,16,17-octahydrocyclopenta[a]phenanthren-3-one
4,5-Secocholest-6-en-4-oic acid, 5-oxo-
Epitestosterone, o-ethyloxime
3-ALPHA-UREIDO-ANDROST-4-EN-17-BETA-OL
Testosterone methoxime, 1.Isomer
Testosterone, oxime
3-METHYLENE-ANDROST-4-EN-17-BETA-OL
Epitestosterone, methyl ether
17.beta.-Hydroxy-4-propyl-3,4-seco-5-androsten-3-oic acid methyl ester
| Title | Journal or Book | Year |
|---|---|---|
| Hydroxylation of steroids by Fusarium oxysporum, Exophiala jeanselmei and Ceratocystis paradoxa | Steroids | 2011 |
| Microbial hydroxylation of steroids. 9. Epoxidation of Δ6-3-ketosteroids by Rhizopusarrhizus ATCC 11145, and the mechanism of the 6β hydroxylase enzyme | Canadian Journal of Chemistry | 1984 |
This compound is available in the following databases:
KnowItAll NMR Spectral Library
Author: Wiley
The KnowItAll NMR Spectral Library offers a comprehensive collection of NMR reference spectra, including NMR, CNMR, and XNMR, and covers a wide range of compounds including organics, polymers, monomers, metabolites, and more. Learn more.
KnowItAll IR, Raman, and UV-Vis Spectral Libraries
Author: Wiley
The KnowItAll IR, Raman, and UV-Vis spectral libraries offer access to the world's largest collection of IR, Raman, and UV-Vis spectra, including the renowned Sadtler spectra. The libraries include a wide range of compounds from pure organics to industrial compounds. Learn more.
KnowItAll Mass Spectral Library
Author: Wiley
The KnowItAll Mass Spectral Library offers a comprehensive collection of mass spectra, including the renowned Wiley Registry, and access to a wide range of compounds including pure organics, drugs, steroids, additives, geochemicals, petrochemicals, biomarkers, and more. Learn more.