6β-Hydroxyandrostenedione
Compound with spectra: 3 NMR, 1 FTIR, and 3 MS (GC)
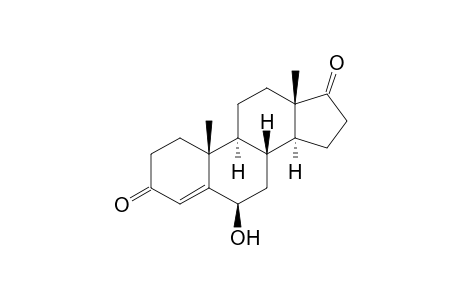
| SpectraBase Compound ID | 85jXQOP7MkC |
|---|---|
| InChI | InChI=1S/C19H26O3/c1-18-7-5-11(20)9-15(18)16(21)10-12-13-3-4-17(22)19(13,2)8-6-14(12)18/h9,12-14,16,21H,3-8,10H2,1-2H3/t12-,13-,14-,16+,18+,19-/m0/s1 |
| InChIKey | WVAMBAWFDOYFOD-DQXCSHPPSA-N |
| Mol Weight | 302.41 g/mol |
| Molecular Formula | C19H26O3 |
| Exact Mass | 302.188195 g/mol |
| Enantiomer InChIKey | WVAMBAWFDOYFOD-RITAXZTOSA-N |
- 4-Androsten-6β-ol-3,17-dione
- 6beta-hydroxyandrost-4-ene-3,17-dione
- 6-BETA-HYDROXYANDROST-4-EN-3,17-DIONE
- 6-Hydroxyandrost-4-ene-3,17-dione
- ANDROST-4-ENE-6.BETA.-OL-3,17-DIONE
- (6R,8R,9S,10R,13S,14S)-6-hydroxy-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,17(2H,6H)-dione
6a-Methyl-androst-4-ene-3,17-dione
6-BETA-CHLORO-ANDROSTAN-4-ENE-3,17-DIONE
6α-bromoandrost-4-ene-3,17-dione
6β-bromoandrost-4-ene-3,17-dione
6-ALPHA-FLUORO-ANDROSTAN-4-ENE-3,17-DIONE
6-BETA-FLUORO-ANDROSTAN-4-ENE-3,17-DIONE
Androst-4-en-17-one, 3,6-dihydroxy-, (3.alpha.,6.beta.)-
6-BETA,17-BETA-DIHYDROXYANDROST-4-EN-3-ONE
3alpha-hydroxy-6alpha-methylandrost-4-en-17-one
ANDROST-4-ENE-6.BETA.-OL-3-ONE(6.BETA.-TRIMETHYLSILYL ETHER)
| Title | Journal or Book | Year |
|---|---|---|
| An unusual ring—A opening and other reactions in steroid transformation by the thermophilic fungus Myceliophthora thermophila | The Journal of Steroid Biochemistry and Molecular Biology | 2009 |
| Predominant allylic hydroxylation at carbons 6 and 7 of 4 and 5-ene functionalized steroids by the thermophilic fungus Rhizomucor tauricus IMI23312 | The Journal of Steroid Biochemistry and Molecular Biology | 2008 |
| Microbial transformation of steroids: Contribution to 14α-hydroxylations | Steroids | 1995 |