Yohimbine
Compound with spectra: 7 NMR, 3 FTIR, 1 Raman, and 6 MS (GC)
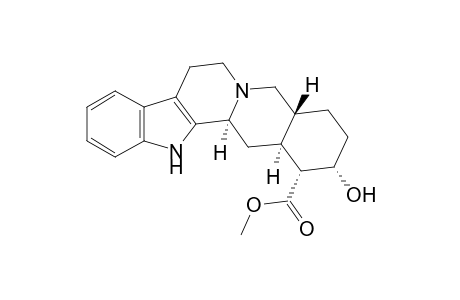
| SpectraBase Compound ID | 5nrjDA6vgV1 |
|---|---|
| InChI | InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 |
| InChIKey | BLGXFZZNTVWLAY-SCYLSFHTSA-N |
| Mol Weight | 354.45 g/mol |
| Molecular Formula | C21H26N2O3 |
| Exact Mass | 354.194343 g/mol |
| Enantiomer InChIKey | BLGXFZZNTVWLAY-RWULDMNWSA-N |
- Corynanthin
- Corynanthine
- Rauhimbin
- Rauhimbine
- Coynanthine
- Methyl (1S,15R,18S,19R,20S)-18-hydroxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
- Methyl (1S,15R,18S,19R,20S)-18-oxidanyl-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate
- Methyl (1R,2S,4aR,13bS,14aS)-2-hydroxy-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydroindolo[2',3':3,4]pyrido[1,2-b]isoquinoline-1-carboxylate
- Methyl (16alpha,17alpha)-17-hydroxyyohimban-16-carboxylate
- Yohimban-16-carboxylic acid, 17-hydroxy-, methyl ester, (16.beta.,17.alpha.)-
- Yohimban-16.beta.-carboxylic acid, 17.alpha.-hydroxy-, methyl ester
Pseudo-yohimbine
Yohimbine
17-epi-alloyohimbine
BLGXFZZNTVWLAY-UHFFFAOYSA-N
.alpha.-Yohimbine
(+-)-3-epialloyohimbine
(+)-BETA-YOHIMBINE
3-EPI-ALPHA-YOHIMBINE
Corynanthine
3-EPI-BETA-YOHIMBINE;(3-BETA,16-ALPHA,17-BETA)-YOHIMBAN-16-CARBOXYLIC-ACID-17-HYDROXY-METHYLESTER
Allo-yohimbine
Yohimbine - isomer
(+-)-3-epi-17-epi-alloyohimbine
Yohimbine II
Benz[g]indolo[2,3-a]quinolizine, yohimban-16-carboxylic acid deriv.
Corynanthine
Yohimban-16-carboxylicacid,17-hydroxy-,methyl ester
| Title | Journal or Book | Year |
|---|---|---|
| Chemical Composition of Aspidosperma ulei Markgr. and Antiplasmodial Activity of Selected Indole Alkaloids | Molecules | 2013 |
| Hydroxylation of Yohimbine in Superacidic Media: One-Step Access to Human Metabolites 10 and 11-Hydroxyyohimbine | Journal of Natural Products | 2001 |
| A Reinvestigation of the Oxidative Rearrangement of Yohimbane-Type Alkaloids. Part A. Formation of pseudoindoxyl ( = 1,2-dihydro-3H-indol-3-one) derivatives | Helvetica Chimica Acta | 1994 |
| 17α-O-Methylyohimbine and vallesiachotamine from roots ofAmsonia elliptica | Phytochemistry | 1990 |
| General methods of synthesis of indole alkaloids. 14. Short routes of construction of yohimboid and ajmalicinoid alkaloid systems and their carbon-13 nuclear magnetic resonance spectral analysis | Journal of the American Chemical Society | 1976 |
This compound is available in the following databases:
Wiley Registry of Tandem Mass Spectral Data: MS for ID
Author: Herbert Oberacher, Institute of Legal Medicine, Innsbruck/Austria
The Wiley Registry® of Tandem Mass Spectral Data: MS for ID contains 10,000 positive and negative mode spectra of over 1200 compounds of interest for forensics, toxicology, and pathology. Learn more.
Sigma-Aldrich Library of Raman Spectra
Author: Wiley/Sigma-Aldrich
Wiley, on behalf of Merck KGaA Darmstadt, Germany, releases the Sigma-Aldrich Library of Raman Spectra, featuring over 6,480 high-quality Raman spectra. Learn more.
Mass Spectra of Designer Drugs 2024
Author: Peter Rösner
This library enables fast and reliable identification of the latest illegal designer drugs. It is updated annually. Learn more.
Maurer, Meyer, Pfleger, Weber: GC-MS Library of Drugs, Poisons, and Their Metabolites 6th Edition
Author: Hans H. Maurer, Markus Meyer, Karl Pfleger, Armin A. Weber
Identification of trace drugs, poisons, and pollutants requires broad coverage of specific compound classes: metabolites, acetylated, methylated, trimethylsilylated, trifluoroacetylated, pentafluoropropionylated, and heptafluorobutyrylated compounds. Learn more.
Wiley Registry of Mass Spectral Data 2023
Author: Wiley
The foundation library for any analytical laboratory running mass spectrometry, the Wiley Registry provides the broadest coverage available in any mass spectral library Learn more.