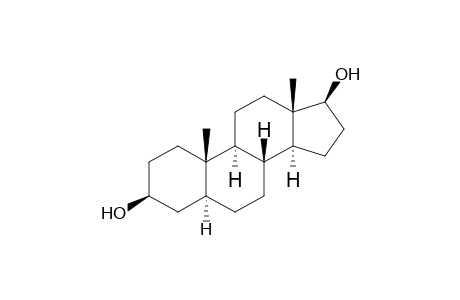
17β-Dihydroepiandrosterone
Compound with spectra: 6 NMR, 2 FTIR, 1 Raman, and 3 MS (GC)
| SpectraBase Compound ID | 2Vx5Zb8JUXV |
|---|---|
| InChI | InChI=1S/C19H32O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12-17,20-21H,3-11H2,1-2H3/t12-,13-,14-,15-,16-,17-,18-,19-/m0/s1 |
| InChIKey | CBMYJHIOYJEBSB-YSZCXEEOSA-N |
| Mol Weight | 292.5 g/mol |
| Molecular Formula | C19H32O2 |
| Exact Mass | 292.24023 g/mol |
| Enantiomer InChIKey | CBMYJHIOYJEBSB-IMECAPTFSA-N |
- 5α-Androstan-3β,17β-diol
- 5a-Androstane-3b,17b-diol
- 5α-Androstan-3α,17β-diol
- 3.beta.,17.beta.-dihydroxy-5.alpha.-androstane
- Androstane-3,17-diol
- (3S,5S,8R,9S,10S,13S,14S,17S)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol
5-ALPHA-ANDROSTAN-3-BETA,17-ALPHA-DIOL
5b-Androstane-3b,17b-diol
17β-Dihydroandrosterone
5β-Androstan-3α,17β-diol
Androstane-3,17-diol
5β-Androstan-3β,17β-diol
Androstane-3,17-diol
5a-Androstane-3a,17b-diol
5b-Androstane-3a,17b-diol
3.ALPHA.,17.BETA.-DIHYDROXY-5.ALPHA.-ANDROSTAN
5-ALPHA-ANDROSTAN-3-ALPHA,17-ALPHA-DIOL
Androstane-3,17-diol
(3-BETA,17A-BETA)-DIHYDROXY-D-HOMOANDROSTAN
3.beta.-Hydroxy-17.beta.-methyl-5a-androstane
5.beta.-androst-16-en-3.alpha.-ol
3B-TRIMETHYLSILOXY-5A-ANDROSTANE
5.beta.-androstane-3.alpha.-ol-trimethylsilyl ether
(2-BETA,17A-BETA)-DIHYDROXY-D-HOMOANDROSTAN
D-Homo-androstane-2b,17ab-diol
No Name
17-(trans-2-Iodoethenyl)-5.alpha.-androstane
Androstan-3-one, 17-iodo-, (17.alpha.)-
| Title | Journal or Book | Year |
|---|---|---|
| Transformation of a series of saturated isomeric steroidal diols by Aspergillus tamarii KITA reveals a precise stereochemical requirement for entrance into the lactonization pathway | The Journal of Steroid Biochemistry and Molecular Biology | 2010 |
| An unusual ring—A opening and other reactions in steroid transformation by the thermophilic fungus Myceliophthora thermophila | The Journal of Steroid Biochemistry and Molecular Biology | 2009 |
| 13C n.m.r. spectra of steroids —a survey and commentary | Organic Magnetic Resonance | 1977 |
| 10.1186/1752-153x-7-164 | "" | "" |