Ergosta-5,24(28)-diene-3.beta.,7.alpha.-diol
Compound with spectra: 6 NMR, and 2 MS (GC)
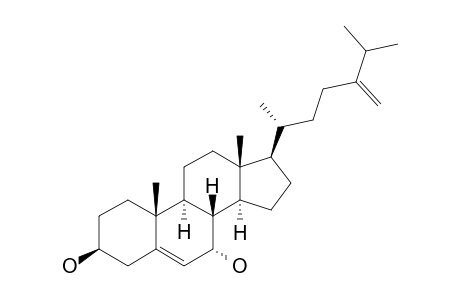
| SpectraBase Compound ID | 2JjtNYsFFna |
|---|---|
| InChI | InChI=1S/C28H46O2/c1-17(2)18(3)7-8-19(4)22-9-10-23-26-24(12-14-28(22,23)6)27(5)13-11-21(29)15-20(27)16-25(26)30/h16-17,19,21-26,29-30H,3,7-15H2,1-2,4-6H3/t19-,21+,22-,23+,24+,25-,26+,27+,28-/m1/s1 |
| InChIKey | OIBDKISTMGYAJC-JEPZXVLISA-N |
| Mol Weight | 414.7 g/mol |
| Molecular Formula | C28H46O2 |
| Exact Mass | 414.349781 g/mol |
| Enantiomer InChIKey | OIBDKISTMGYAJC-GVJNLOTNSA-N |
- 24-METHYLENE-5-CHOLESTEN-3-BETA,7-ALPHA-DIOL
- 3-BETA,7-ALPHA-DIHYDROXYERGOSTA-5,24(28)-DIENE
- 3-BETA,7-ALPHA-DIHYDROXYERGOST-5,24(28)-DIENE
- Sterol
(24Z)-STIGMASTA-5,24(28)-DIENE-3-BETA,7-ALPHA-DIOL
(6R)-6-[(3S,7S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-3,7-bis(oxidanyl)-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-methyl-heptan-3-one
Cholest-5-ene-3,7-diol, (3.alpha.,7.alpha.)-
Cholest-5-ene-3,7-diol, (3beta)-
DECORTINOL;STIGMASTA-5,25-DIEN-3-BETA,7-ALPHA-DIOL
ISODECORTINOL
| Title | Journal or Book | Year |
|---|---|---|
| Synthesis and Structure-Activity Relationships of Cytotoxic 7-Hydroxy Sterols | Journal of Natural Products | 1994 |
| Marine Sterols. Side-Chain-Oxygenated Sterols, Possibly of Abiotic Origin, from the New Caledonian Sponge Stelodoryx chlorophylla | Journal of Natural Products | 1993 |
| Bioactive Ergost-5-ene-3β/,7α-diol Derivatives from Pseudobersama mossambicensis | Journal of Natural Products | 1992 |
| An ergostane derivative from the bark of Entandrophragma utile | Phytochemistry | 1992 |
| Complete Structural Assignments of an Ergosterol Derivative from Entandrophragma utile | Journal of Natural Products | 1991 |
| Novel sterols from the finger sponge Haliclonaoculata | Canadian Journal of Chemistry | 1985 |
This compound is available in the following databases:
Wiley Registry of Mass Spectral Data 2023
Author: Wiley
The foundation library for any analytical laboratory running mass spectrometry, the Wiley Registry provides the broadest coverage available in any mass spectral library Learn more.